The mechanisms discovered through the study of embryonic development have been fundamental to understanding disease. The Kurpios lab in the Department of Molecular Medicine uses a combination of classical chicken embryology and modern mouse genetics to elucidate how basic cellular processes define the shape and function of organs. We are most fascinated by the evolutionarily conserved left-right (LR) organ asymmetry as errors of organ laterality (reversed or randomized growth of organs) are fundamentally linked to life-threatening birth defects, highlighting an urgent need to define the molecular 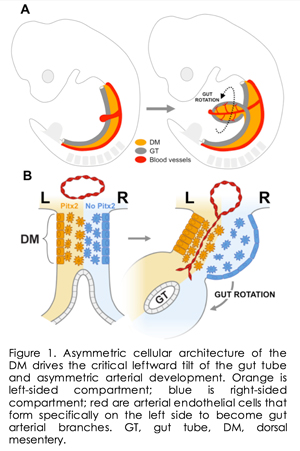 basis of organ asymmetry. basis of organ asymmetry.
The counterclockwise rotation of the gut (Figure 1A) serves as an excellent model to study LR asymmetry. A critical aspect of this rotation is initiation of a leftward tilt directed by the conserved Pitx2 transcription factor, the master regulator of LR organ asymmetry. Failure to establish proper gut chirality leads to gut malrotation and catastrophic volvulus in pediatric patients and domestic animals. The direction of gut rotation has long been assumed to be intrinsic to the tube itself. Our research, however, has challenged this paradigm and demonstrated that rotation is instead driven by asymmetric cellular behavior within the dorsal mesentery (DM, Figure 1B), a mesodermal structure that suspends the gut tube and whose cellular architecture is downstream of Pitx2 expressed strictly on the DM left side. A key property of the DM is its exquisite binary cellular organization, with distinct left and right compartments that are readily accessible to genetic manipulation. Cellular and extracellular matrix (ECM) changes in each compartment cause the DM to deform and subsequently tilt the attached gut tube leftward.This critical leftward bias determines subsequent gut chirality and provides a framework for understanding how LR asymmetry in gene expression is ultimately responsible for changes in cell behavior that initiate asymmetric organogenesis.
We were the first toperform laser capture microdissection and genom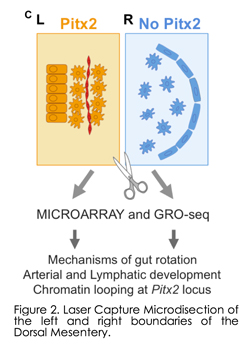 e-wide sequencing of the left (Pitx2 positive) and right (Pitx2 negative) DM compartments (Fig. 2) and have since pioneered this tissue as a highly tractable in vivo model to study organogenesis at the level of cell shape, adhesion, ECM, actin cytoskeleton, effector protein function and most recently vascular development and chromatin structure (Dev Cell 2008, PNAS 2008, Nature 2011, Dev Cell 2013, Dev Cell 2014, Cell Reports 2015, Dev Cell 2018). While our initialeffort focused on the identification of molecular and cellular targets of Pitx2, our research unexpectedly revealed that vascular development of gut arteries is commensurate with the onset of gut rotation and proceeds strictly on the DM left side dependent on Pitx2, whereas all vasculature is inhibited on the right side at the onset of rotation. Indeed, in addition to directing gut rotation, the DM is the major conduit for extensive blood and lymphatic vessels that nourish and drain the digestive tract but the mechanisms underlying morphogenesis of these e-wide sequencing of the left (Pitx2 positive) and right (Pitx2 negative) DM compartments (Fig. 2) and have since pioneered this tissue as a highly tractable in vivo model to study organogenesis at the level of cell shape, adhesion, ECM, actin cytoskeleton, effector protein function and most recently vascular development and chromatin structure (Dev Cell 2008, PNAS 2008, Nature 2011, Dev Cell 2013, Dev Cell 2014, Cell Reports 2015, Dev Cell 2018). While our initialeffort focused on the identification of molecular and cellular targets of Pitx2, our research unexpectedly revealed that vascular development of gut arteries is commensurate with the onset of gut rotation and proceeds strictly on the DM left side dependent on Pitx2, whereas all vasculature is inhibited on the right side at the onset of rotation. Indeed, in addition to directing gut rotation, the DM is the major conduit for extensive blood and lymphatic vessels that nourish and drain the digestive tract but the mechanisms underlying morphogenesis of these 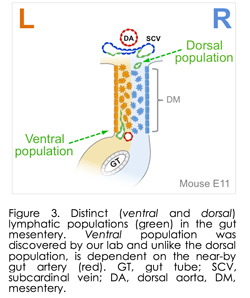 channels are unknown. channels are unknown.
Importantly, we discovered substantial errors in the standard textbook accounts of the origins and remodeling of mesenteric lymphatic vessels, which are among the largest in the body and defects of which cause debilitating intestinal metabolic dysfunctions such as inflammatory bowel disease and obesity. Indeed, after more than a 100-year debate over the origin of the lymphatic vessels, it is well accepted that lymphatic cells sprout from veins. Our laboratory has challenged this dogma with our discovery that lymphatic development in the gut depends on arteries, independent of veins. Thus, we found a new population of immature lymphatic progenitor cells that specifically form the intestinal endothelial lymphatic vessels. Published in Developmental Cell in 2014 (and winning us the journal cover art), this finding is important because gut lymphatics are involved in many debilitating disorders, including lymphedema, lymphangiectasia, inflammatory bowel disease, and obesity. This discovery paves the way to develop therapies that might specifically target gut lymphatics, leaving others throughout the body unaffected. We now propose targeted intervention of lymphatic progenitor cells in the gut mesentery, the tissue that suspends the gut tube along its entire length. The gut mesentery is the tissue of origin of two heterogeneous lymphatic precursor cell populations we have identified 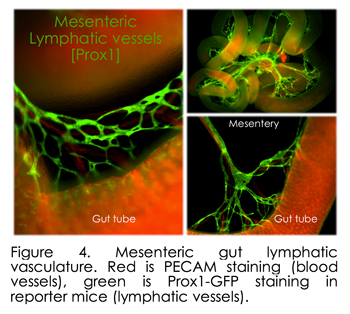 (dorsal and ventral populations, Figure 3). Very recent papers from other laboratories confirmed heterogeneous lymphatic subsets within the skin and the heart (Circulation 2015, Nature 2015 [mouse]; Nature 2015 [zebrafish]), raising challenging questions about the overall functional heterogeneity among lymphatics, a system correlated with multiple diseases of unknown etiology. However, gut lymphatics are among the largest in the body and serve as the sole and essential channels for absorption and transport of dietary lipids, including fat-soluble vitamins, a function that separates them from all other lymphatic networks (Figure 4). In addition to their essential, life-supporting roles, lymphatic channels are the primary conduits for metastatic spread of colorectal tumor cells and lymphatic defects cause a wide range of debilitating intestinal metabolic dysfunctions. However, the molecular mechanisms governing their specialized functions remain unknown. What the field is missing are a set of genes expressed in various lymphatic subsets that distinguish them from other lymphatics, and can be used to drive reporter genes or recombinases to compromise candidate gene function in the mouse. Our lab is pursuing these questions using stat of the art single-cell RNA sequencing. (dorsal and ventral populations, Figure 3). Very recent papers from other laboratories confirmed heterogeneous lymphatic subsets within the skin and the heart (Circulation 2015, Nature 2015 [mouse]; Nature 2015 [zebrafish]), raising challenging questions about the overall functional heterogeneity among lymphatics, a system correlated with multiple diseases of unknown etiology. However, gut lymphatics are among the largest in the body and serve as the sole and essential channels for absorption and transport of dietary lipids, including fat-soluble vitamins, a function that separates them from all other lymphatic networks (Figure 4). In addition to their essential, life-supporting roles, lymphatic channels are the primary conduits for metastatic spread of colorectal tumor cells and lymphatic defects cause a wide range of debilitating intestinal metabolic dysfunctions. However, the molecular mechanisms governing their specialized functions remain unknown. What the field is missing are a set of genes expressed in various lymphatic subsets that distinguish them from other lymphatics, and can be used to drive reporter genes or recombinases to compromise candidate gene function in the mouse. Our lab is pursuing these questions using stat of the art single-cell RNA sequencing.
More recently, we shifted are focus from the Pitx2 dominated left side of the dorsal mesentery to the right side of this structure (blue in Figure 1). Indeed, for many years, biologists including our, lab have focused on the role o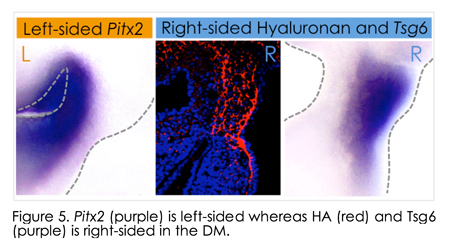 f the left-sided Pitx2 in governing organ laterality. However, after careful analyses in chicken and mouse embryos, we recently learned that morphological asymmetry in the DM is first broken on the right side of the DM, not the left. Unexpectedly, this LR symmetry-breaking event depends on the accumulation of hyaluronan (HA), a major component of the extracellular matrix (ECM), and is, surprisingly, independent of Pitx2 activity on the left. Whereas HA is synthesized bilaterally in the DM, we show that on the right side HA is covalently modified with heavy chain (HC) peptides by the enzyme Tsg6 (tumor necrosis factor α-stimulated gene 6), resulting in asymmetric accumulation of stable HC-HA matrices on the right side. HC-HA then triggers dramatic expansion of the right side of the DM necessary for midgut rotation and gut vascular development, linking vessel patterning with the morphogenesis of the host organ. HA disruption in chicken f the left-sided Pitx2 in governing organ laterality. However, after careful analyses in chicken and mouse embryos, we recently learned that morphological asymmetry in the DM is first broken on the right side of the DM, not the left. Unexpectedly, this LR symmetry-breaking event depends on the accumulation of hyaluronan (HA), a major component of the extracellular matrix (ECM), and is, surprisingly, independent of Pitx2 activity on the left. Whereas HA is synthesized bilaterally in the DM, we show that on the right side HA is covalently modified with heavy chain (HC) peptides by the enzyme Tsg6 (tumor necrosis factor α-stimulated gene 6), resulting in asymmetric accumulation of stable HC-HA matrices on the right side. HC-HA then triggers dramatic expansion of the right side of the DM necessary for midgut rotation and gut vascular development, linking vessel patterning with the morphogenesis of the host organ. HA disruption in chicken 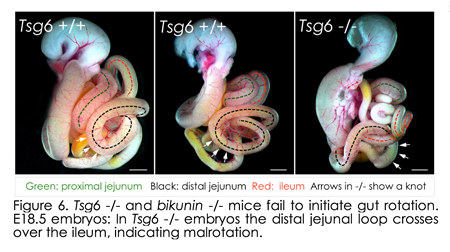 and Tsg6 -/- mice results in failure to initiate midgut rotation and perturbs vascular development predisposing to midgut volvulus. and Tsg6 -/- mice results in failure to initiate midgut rotation and perturbs vascular development predisposing to midgut volvulus.
Thus, our study leads us to revise the current symmetry-breaking paradigm in vertebrates and demonstrates how enzymatic modification of HA matrices can execute the blueprint of organ laterality. Moreover, our studies implicate the embryo’s right side as an active - rather than a passive “not left” - compartment and provide new mechanistic insights into the role of HA matrices during asymmetric organ morphogenesis.
Collectively, our current research is organized in the following three areas: 1) Mechanisms underlying asymmetric gut rotation and vascular remodeling; 2) Signaling pathways involving lymphatic development; 3) Chromatin level mechanisms at the Pitx2 locus.
Please contact natasza.kurpios@cornell.edu if you are interested in joining our team!
|